Cyclodextrins are non-reducing cyclic glucose oligosaccharides.
Cyclodextrins result from the cyclomaltodextrin glucanotransferase catalyzed degradationof starch.
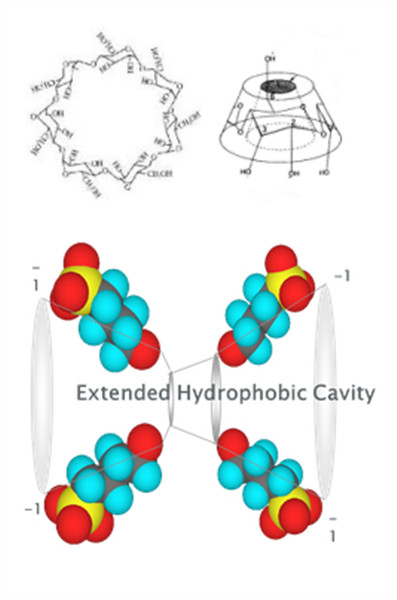
Their structures and use in the food industry have been reviewed. There are three common cyclodextrins with 6, 7 or 8 D-glucopyranonsyl residues (α-, β-, γ-cyclodextrin respectively) linked in a ring by α-1,4 glycosidic bonds. The glucose residues have the4C1 conformation. All three cyclodextrins have similar structures (that is, bond lengths and orientations) apart from the structural necessities of accommodating a different number of glucose residues. They present a bottomless bowl-shaped (truncated cone) molecule stiffened by hydrogen bonding between the 3-OH and 2-OH groups around the outer rim. The hydrogen bond strengths are α-cyclodextrin < β-cyclodextrin < γ-cyclodextrin. Cyclodextrins are non-reducing cyclic glucose oligosaccharides Cyclodextrins result from the cyclomaltodextrin glucanotransferase catalyzed degradation of starch. Their structures and use in the food industry have been reviewed. There are three common cyclodextrins with 6, 7 or 8 D-glucopyranonsyl residues (α-, β-, γ-cyclodextrin respectively) linked in a ring by α-1,4 glycosidic bonds. The glucose residues have the4C1 conformation. All three cyclodextrins have similar structures (that is, bond lengths and orientations) apart from the structural necessities of accommodating a different number of glucose residues. They present a bottomless bowl-shaped (truncated cone) molecule stiffened by hydrogen bonding between the 3-OH and 2-OH groups around the outer rim. The hydrogen bond strengths are α-cyclodextrin < β-cyclodextrin < γ-cyclodextrin.
|
